Earth
ID: 826
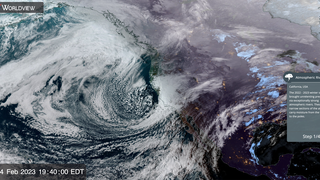
Most stratospheric chlorine comes from man-made compounds called chlorofluorocarbons or CFCs. CFCs, widely used in refrigerators and air conditioners, are quite harmless and non-reactive in the lower atmosphere. Carried slowly upward by the earth's winds, they can survive the 5 year journey to the upper stratosphere. Here, above most of the ozone layer, the sun's ultraviolet radiation breaks down the CFCs into the more reactive chlorine compounds that destroy ozone. Chlorine can react with methane to form hydrogen chloride. Chlorine can also react with ozone forming the radical chlorine monoxide. Chlorine monoxide then combines with the radical nitrogen dioxide to form stable chlorine nitrate. Chlorine nitrate and hydrogen chloride are called reservoir gases for the chlorine radical. These reservoir gases usually contain more than ninety percent of the chlorine in the lower stratosphere.

Chemical Model Animation of CFCs Releasing Chlorine to Form Reservoir Gases

Used Elsewhere In
For More Information
Visualization Credits
James W. Williams (GST): Lead Animator
Jesse Allen (Raytheon): Animator
Greg Shirah (NASA/GSFC): Animator
Mark Schoeberl (NASA/GSFC): Scientist
Jesse Allen (Raytheon): Animator
Greg Shirah (NASA/GSFC): Animator
Mark Schoeberl (NASA/GSFC): Scientist
Please give credit for this item to:
NASA/Goddard Space Flight Center Scientific Visualization Studio
NASA/Goddard Space Flight Center Scientific Visualization Studio
Short URL to share this page:
https://svs.gsfc.nasa.gov/826
This item is part of this series:
UARS
Keywords:
SVS >> Chlorofluorocarbons
NASA Science >> Earth
https://svs.gsfc.nasa.gov/826
This item is part of this series:
UARS
Keywords:
SVS >> Chlorofluorocarbons
NASA Science >> Earth