Original SOLVE
Campaign Observes
|
 |
 |
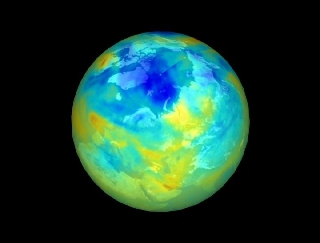
| Ozone Hole | Polar Stratospheric Clouds |
| Read the official press
release April 2000 |
Read the official
press release May 30, 2000 |
| (Released at Spring 2000 American Geophysical Union (AGU) Meeting) |
During the winter of 1999-2000 Arctic ozone levels reached their lowest point in eight years at an altitude of nearly 60,000 feet. Concentrations dropped more than 50 percent from their average. But measurements taken during the largest international campaign ever mustered to study the Arctic stratosphere are yielding better insights into the processes that control polar ozone. Called SOLVE (Stratospheric Ozone Loss and Validation Experiment), it included researchers from Europe, Russia, Canada, and the United States working together to develop better tools for predicting the state of polar ozone levels. These predictive tools will become more and more important in light of expected chlorine level declines due to the Montreal Protocol and what will likely be increasing levels of greenhouse gases in the coming decades.
A Gap in the Churning Sky

View Images and Movies
A combination of factors contributed to the dramatic drop in Arctic stratosphere ozone levels. Despite the very dry conditions in the polar stratosphere, temperatures plunged far enough to enable the formation of Polar Stratospheric Clouds (PSCs) at an unusually early date. PSCs are considered the primary culprits for ozone losses around the North Pole. By chemically interacting with the surrounding atmosphere, they convert inorganic chlorine contained in non-harmful compounds into "free radicals", ambient forms of chlorine that can interact destructively with ozone.
Research into polar ozone can provide valuable information for people living in more heavily populated regions. As winter wanes and air temperatures rise, the polar vortex begins to break up. Low ozone atmosphere that throughout the winter had been mostly contained by the vortex now begins to propagate into lower latitudes, thus causing average ozone levels to fall at lower latitudes.
Although it is commonly believed that greenhouse gases can cause higher temperatures in the lower atmosphere, or troposphere, studies suggest that rising greenhouse gases contribute to lower temperatures in the upper atmosphere. Scientists are increasingly worried that cooler temperatures in the Arctic atmosphere may lead to increased formation of PSCs, and thereby massive ozone losses. The ability to predict these losses requires detailed understanding of the chemistry, dynamics, and radiative properties. Although it is commonly believed that greenhouse gases can cause higher temperatures in the lower atmosphere, or troposphere, studies suggest that rising greenhouse gases contribute to lower temperatures in the upper atmosphere. Scientists are increasingly worried that cooler temperatures in the Arctic atmosphere may lead to increased formation of PSCs, and thereby massive ozone losses. The ability to predict these losses requires detailed understanding of the chemistry, dynamics, and radiative properties of the
Cold Clouds

View Images and Movies
Polar stratospheric clouds form at extremely low temperatures in the upper atmosphere. Should the temperature rise, clouds won't form. In this visualization, sequential temperature readings taken in the SOLVE research area are plotted against a threshold temperature for PSC formation. These are clouds essentially made of nitric acid. Note how the area covered by the clouds increases as winter progresses. The red point on the map indicates the location of Kiruna, Sweden, the SOLVE staging area.
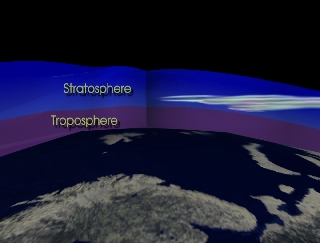
The Polar Vortex
View Images and Movies
During winter in the Northern Hemisphere, stratospheric winds tend to form a vortex around the pole. Measured ozone losses in the winter of 1999-2000 were unusually severe, propelled by cold temperatures and the commensurate formation of Polar Stratospheric Clouds. The atmospheric vortex essentially forms a container for high altitude air to lose ozone due to chemical changes. Measurements of total atmospheric ozone were taken by NASA's high altitude ER-2 aircraft, and the space agency's DC-8. Readings from NASA's Total Ozone Mapping Spectrometer (TOMS) Earth Probe showed a clear ozone minimum over the polar region during February and March.
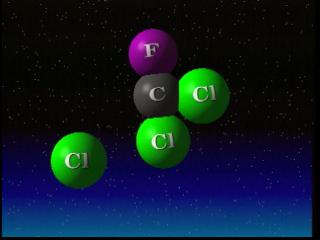
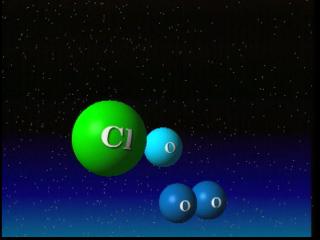
Ozone Loss
 |
 |
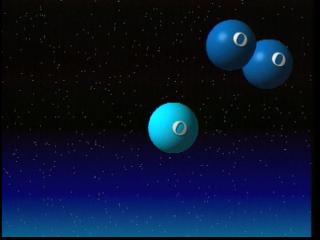 |
Ozone is produced by intense ultraviolet radiation in the upper stratosphere. This radiation breaks typical oxygen molecules (O2) into free oxygen atoms. Those free atoms of oxygen (O) then join with molecular oxygen (O2) and form molecules of ozone (O3). The ozone molecule generally absorbs ultra-violet radiation. But ozone is destroyed when it reacts with one of a variety of chemicals in the stratosphere such as chlorine, nitrogen, bromine or hydrogen.
The process happens essentially in three steps. In step one, an ozone molecule is cracked by sunlight to form an oxygen atom and an oxygen molecule. In step two, a catalyst, in this case chlorine, reacts with another ozone molecule to form ClO and a second oxygen molecule. Finally, the ClO molecule reacts with the oxygen atom to form a third oxygen molecule, and reconstitute the original catalyst. The catalyst converts two ozone molecules into three oxygen molecules without being affected itself. A typical chlorine atom can destroy a large number of ozone molecules in this fashion.
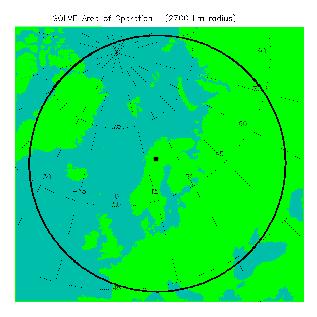
Deployment site
View Images and Movies
In 2000, Kiruna, Sweden was chosen as the deployment site for SOLVE for two reasons.
First, the Arena Arctica facility at the Kiruna airport is a superb hanger
for the ER-2 and DC-8 operations. Second, Kiruna's extreme northern latitude
is ideally located for measurements of the lower stratospheric polar vortex.
The location of Kiruna, Sweden is noted by the black point on the map,
and a 2000 km circle is drawn around town, illustrating the range of both
the ER-2 and DC-8 during this mission. For further information, check
out the following web sites about ongoing polar ice and oceanic research:
Read more stories about Ozone Measurement
This multimedia project is the work of a dedicated team of researchers,
animators, and media specialists. A companion video to this web site is
available from NASA-TV. Below are a list of agencies, departments, and
researchers who provided expertise and data for this production:
Special thanks to
Dr. Paul Newman/Atmospheric Physicist, SOLVE Project Scientist
Please give credit for these images to:
NASA - Goddard Space Flight Center
Scientific Visualization Studio
Television Production NASA-TV/GSFC
Content Preparation and Project Production:
Michael Starobin
Last Revised: February 4, 2019 at 06:02 PM EST